Participant Management
Overview
The Participant Management tab in the study edit page allows researchers to configure settings related to participant enrollment, group assignments, and study policies. This section provides a comprehensive guide to managing participants, ensuring your study runs smoothly and aligns with your research design. From setting study duration to defining recruitment strategies, these settings give you granular control over how participants interact with your study.
Effective participant management is critical for maintaining data quality and participant engagement. The SMAAT platform offers flexible options to accommodate diverse study designs, such as randomized group assignments or custom participant codes, while ensuring compliance with ethical standards through informed consent forms.
Study Length
Specify the duration of the study for each participant in days. This setting determines when event-based notifications, such as a debriefing survey at the study’s end, are triggered.
Example: For a 14-day study on sleep patterns, set the study length to 14 days. A debriefing survey can be scheduled to send automatically on the 14th day after a participant’s enrollment.
Recruitment Strategy
Define the recruitment period by specifying start and end dates. These dates are displayed in the SMAAT app during onboarding and on the study information page, helping participants understand the study’s timeline.
Example: For a study running from January 1 to March 31, 2026, set the recruitment start date to January 1 and the end date to February 28 to allow a buffer for data collection.
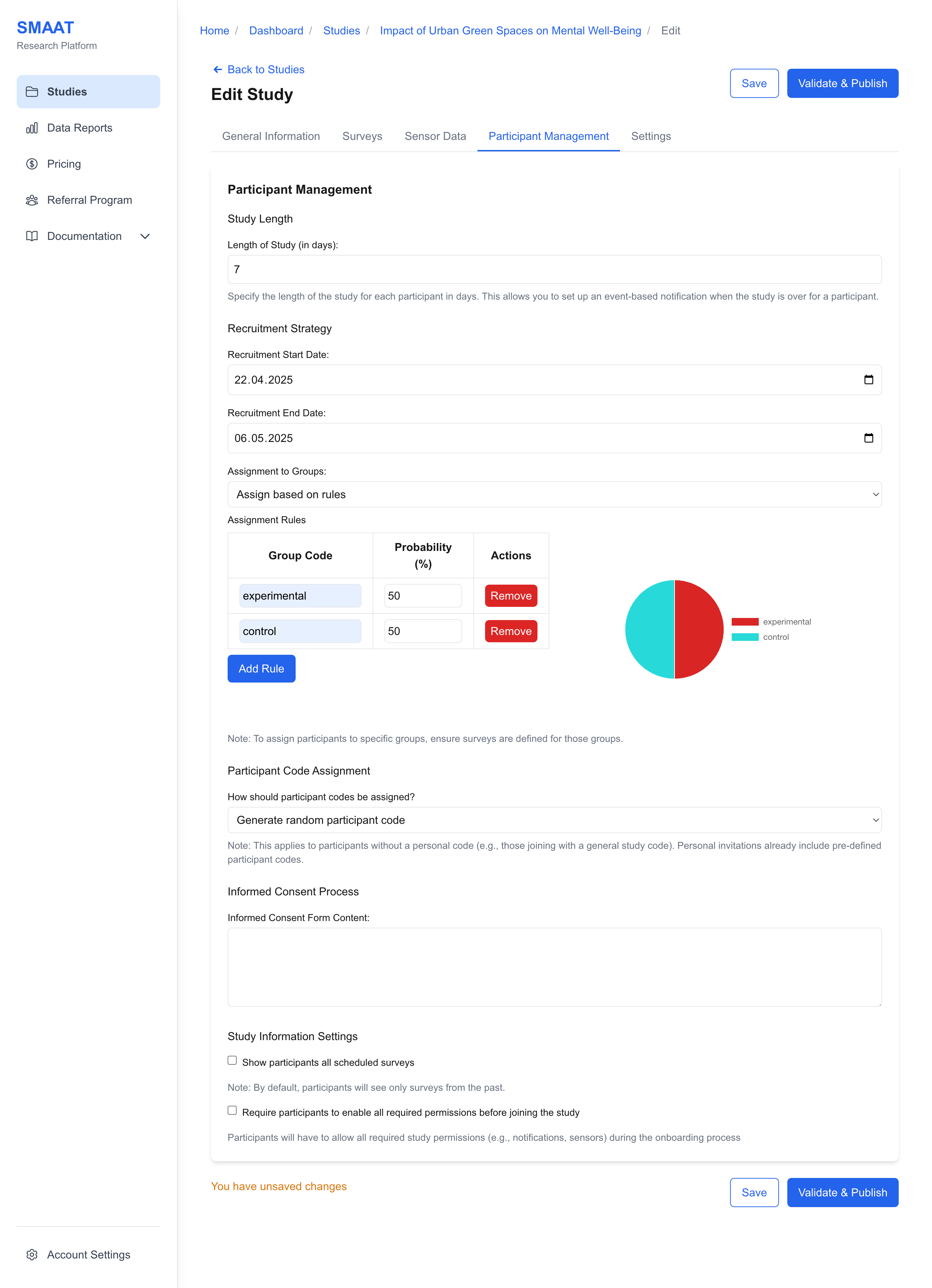
Assignment to Groups
Assign participants to groups to deliver different surveys or conditions. The platform offers several group assignment strategies:
- Do Not Assign: All participants receive the same surveys, with no group differentiation.
- Assign All to a Specific Group: Assign all new participants a specific group code (e.g., “Control”).
- Assign Based on Join Time: Assign group codes based on when participants join (e.g., “Group A” for January joiners, “Group B” for February joiners).
- Assign Based on Rules: Assign group codes probabilistically (e.g., 50% chance for “Group A,” 50% for “Group B”). A pie chart visualizes the distribution.
- Allow Participants to Enter Group Code: Participants input a pre-assigned code, with instructions provided (e.g., “Enter the code provided by your study coordinator”).
- Allow Participants to Select Group Code: Participants choose from a list of group codes, with instructions and options provided.
Example: For a study comparing two interventions, use rule-based assignment with a 50% probability for “Intervention A” and 50% for “Intervention B” to ensure balanced groups.
Troubleshooting Tip: If group assignments are not working as expected, verify the group codes in your survey settings match those assigned to participants.
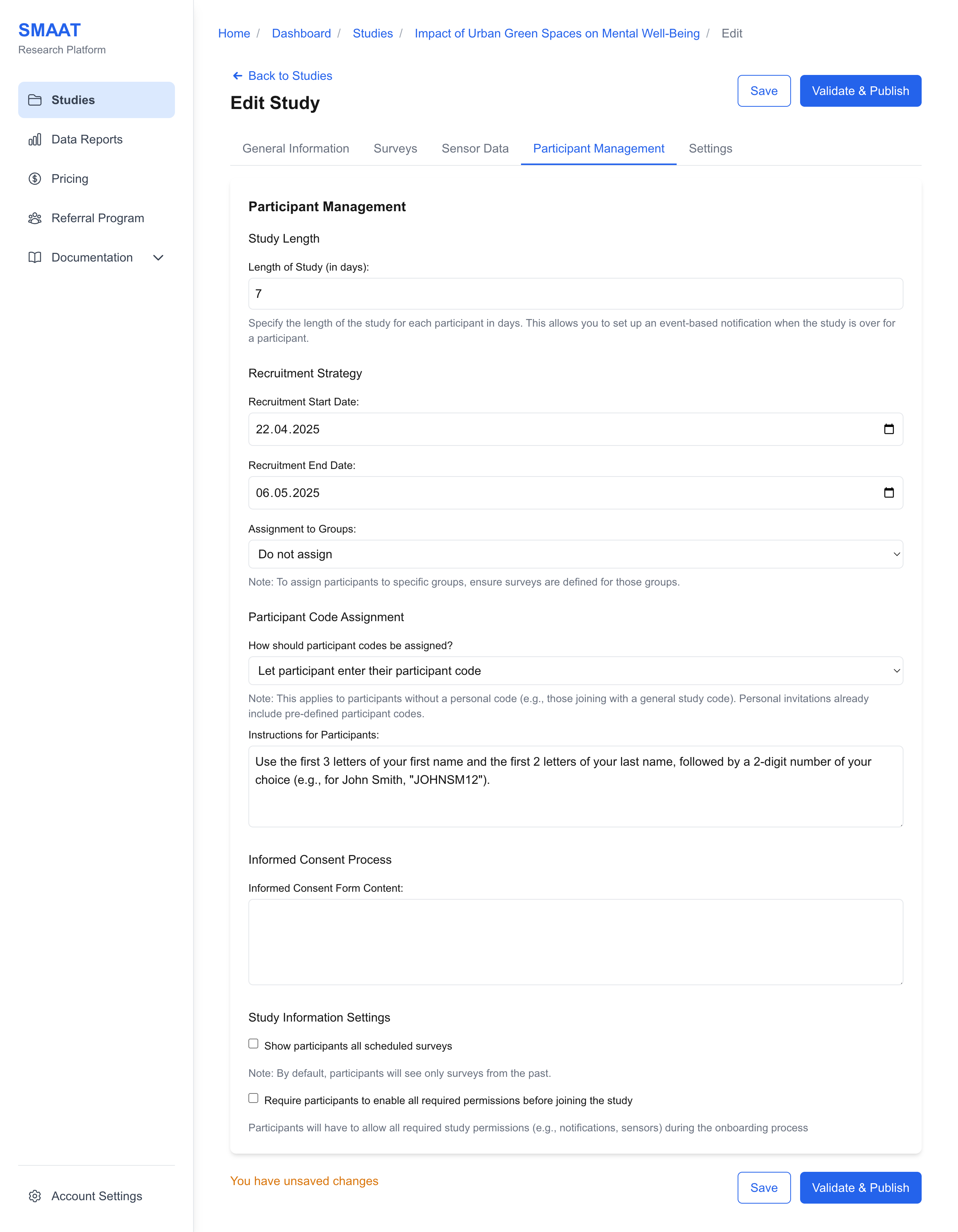
Participant Code Assignment
Assign unique participant codes to identify individuals in your study. Choose from:
- Random Generation: The platform generates random codes (e.g., “P123”) for participants without pre-defined codes.
- Participant-Generated: Instruct participants to create their own codes (e.g., “Use the first two letters of your name and your birth year”).
Note: Participants joining via custom invitations already have pre-defined codes, overriding this setting.
Example: For anonymity, use random generation to assign codes like “P456” to participants joining with a general study code.
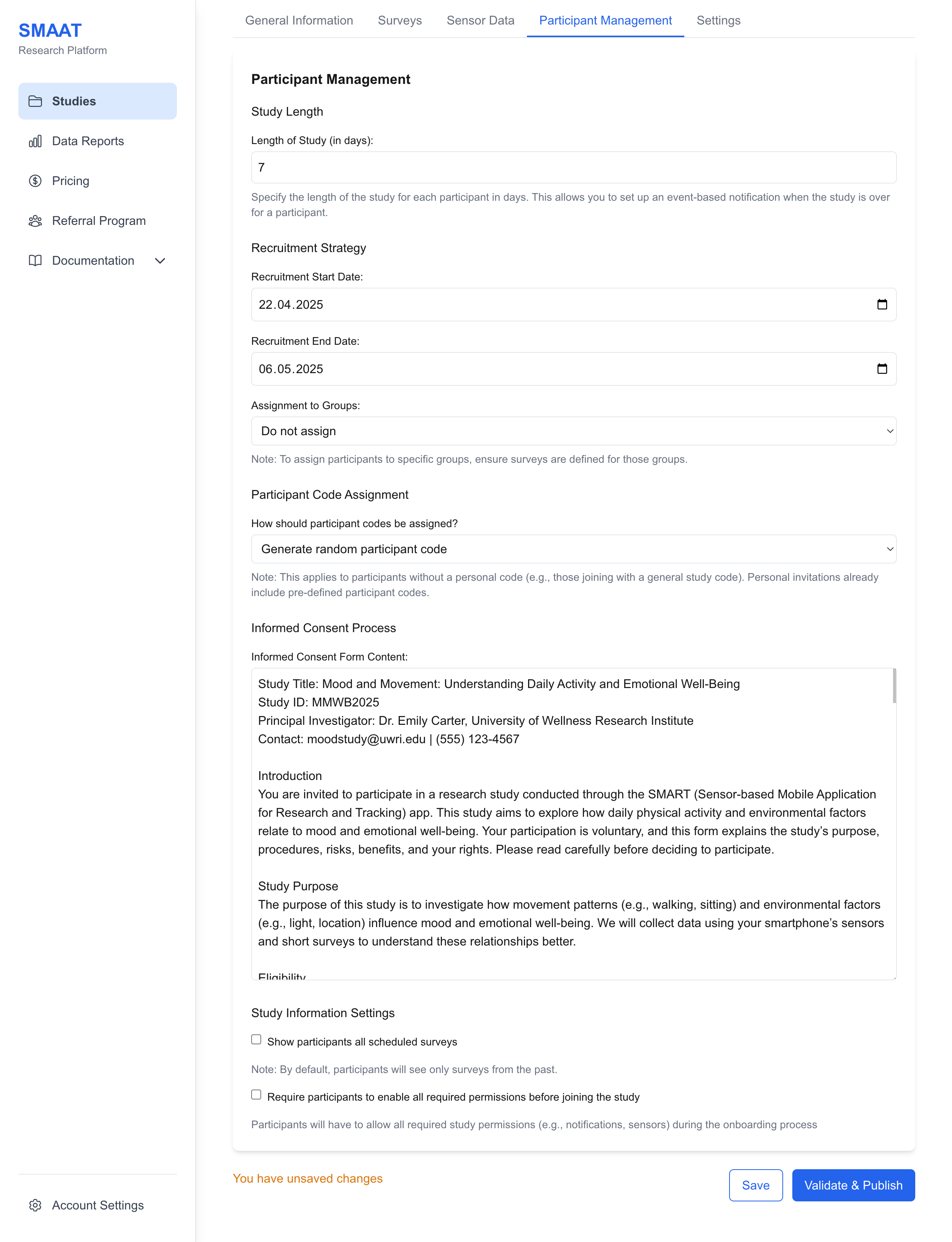
Informed Consent Form
Enter the text for the informed consent form, which participants must agree to during onboarding. This form is a standard ethical requirement and should detail the study’s purpose, procedures, risks, and participant rights.
Example: “By participating in this study, you agree to allow your location and survey data to be collected anonymously for research on urban mobility. You may withdraw at any time without penalty.”
Troubleshooting Tip: Do not leave the consent form blank, as it is required for ethical compliance. If the form is empty, participants may not be able to join the study.
Study Information Settings
Customize how study information is presented to participants:
- Show All Scheduled Surveys: Enable to display all planned surveys and their scheduled times in the app. Disable (default) to show only past surveys, useful for randomized schedules.
- Require Permissions Before Joining: Enable to mandate that participants grant all required permissions (e.g., notifications, sensors) during onboarding. Disable (default) to allow joining without permissions, which participants can enable later.
Example: For a study with random notifications, disable “Show All Scheduled Surveys” to prevent participants from anticipating survey times.
Next Steps
With participant management settings configured, explore the following sections to continue building and managing your research on the SMAART platform:
Study Setup
- Settings: Manage study settings.
Study Execution
- Study Dashboard: Monitor invitations, participants, notifications, and data.
Data Analysis
- Data Analysis: Build visualizations to analyze study data.
- Background Sensor Data: Analyze passive sensor data collected from participants.
- Data Reports: Generate comprehensive reports from study data.
- Frequently Asked Questions: Find answers to common questions about the SMAAT platform.